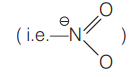Q.
For which of the following. Kjeldahl's method is not used for the estimation of nitrogen?
I. Aniline
II. Azobenzene
III. Nitrobenzene
IV. Pyridine
Solution: