- Tardigrade
- Question
- Chemistry
- For a reaction, consider the plot of logK versus (1/ T) given in the figure. If rate constant of this reaction at text400 K is10- 5sec- 1 then the rate constant at 500K is :- <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-reol0btsvm3jl4tm.jpg />
Q.
For a reaction, consider the plot of versus given in the figure.
If rate constant of this reaction at then the rate constant at is :-
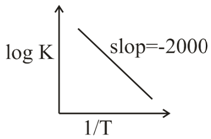
Solution:
