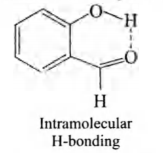
Q. Explain, why o-hydroxy benzaldehyde is a liquid at room temperature while p-hydroxy benzaldehyde is a high melting solid?
Solution:
Intramolecular H-bonding in ortho hydroxy benzaldehyde is
responsible for decrease in melting and boiling points.
p-hydroxy benzaldehyde molecules are associated by
intermolecular H-bonding, has higher melting and boiling
points.