- Tardigrade
- Question
- Chemistry
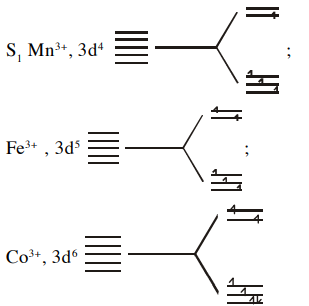
- Consider the following statements: S 1:[ MnCl 6]3-,[ FeF 6]3- and [ CoF 6]3- are paramagnetic having four, five and four unpaired electrons respectively. S2: Valence bond theory gives a quantitative interpretation of the thermodynamic stabilities of coordination compounds. S3: The crystal field splitting Δo, depends upon the field produced by the ligand and charge on the metal ion and arrange in the order of true/ false.
Q.
Consider the following statements :
and are paramagnetic having four, five and four unpaired electrons respectively.
: Valence bond theory gives a quantitative interpretation of the thermodynamic stabilities of coordination compounds.
: The crystal field splitting , depends upon the field produced by the ligand and charge on the metal ion and arrange in the order of true/ false.
Solution: