- Tardigrade
- Question
- Chemistry
- Consider the following statements about the graph given below, <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/1de662febdb2e7c21b691d7a0ef9832f-.png /> I. After a certain time, the composition of the mixture remains same. II. The reaction mixture starting with either H 2 or D 2 reach equilibrium with same composition. III. The constancy in composition indicates that the reaction has reached equilibrium. Choose the correct statements.
Q.
Consider the following statements about the graph given below,
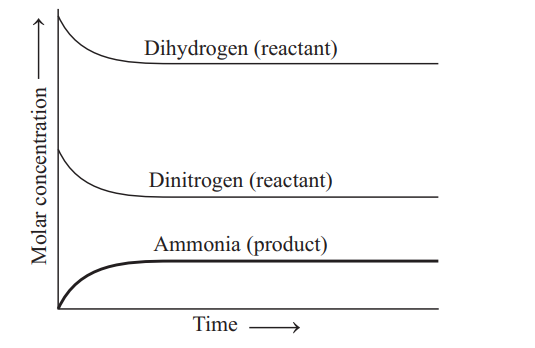
I. After a certain time, the composition of the mixture remains same.
II. The reaction mixture starting with either or reach equilibrium with same composition.
III. The constancy in composition indicates that the reaction has reached equilibrium.
Choose the correct statements.
Solution: