- Tardigrade
- Question
- Chemistry
- Consider the following case of completing 1 st order reactions <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/4e347b42e053bfa16c7196b7fe7ff6bf-.png /> After the start of the reaction at t=0 with only A, the [B] is equal to the [C] at all times. The time in which all three concentrations will be equal is given by
Q.
Consider the following case of completing st order reactions
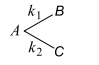
After the start of the reaction at with only , the is equal to the at all times. The time in which all three concentrations will be equal is given by
Solution: