Q.
Consider the following case of completing $1$ st order reactions
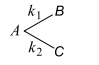
After the start of the reaction at $t=0$ with only $A$, the $[B]$ is equal to the $[C]$ at all times. The time in which all three concentrations will be equal is given by
Chemical Kinetics
Solution: