Q. Calculate the percentage of all the monochlorinated products obtained from the chlorination of 2 - methyl butane. The relative reactivity of , , and hydrogen to chlorination is (1 : 3.8 : 5).
Solution:


There are four different (A, B, C and D) monochloro products.
Relative amount
A

(A) is obtained from the reaction of three equivalent H atoms.
3 x 1 = 3.0
B
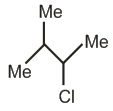
(B) is obtained from the reaction of two equivalent H atoms.
2 x 3.8 = 7.6
C

(C) is obtained from the reaction of one equivalent H atoms.
1 x 5 = 5.0
D

(D) is obtained from the reaction of six equivalent H atoms.
6 x 1 = 6.0

Total = 21.6
Therefore, percentages of the and D monochloro products are as follows:
| Relative amount | |||
|---|---|---|---|
| A |  |
(A) is obtained from the reaction of three equivalent H atoms. | 3 x 1 = 3.0 |
| B |  |
(B) is obtained from the reaction of two equivalent H atoms. | 2 x 3.8 = 7.6 |
| C |  |
(C) is obtained from the reaction of one equivalent H atoms. | 1 x 5 = 5.0 |
| D |  |
(D) is obtained from the reaction of six equivalent H atoms. | 6 x 1 = 6.0 |
 |
Total = 21.6 |