- Tardigrade
- Question
- Chemistry
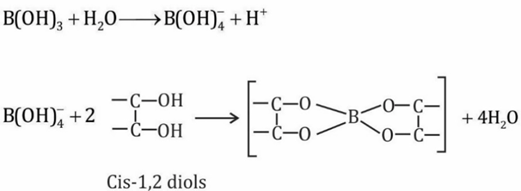
- <math> <mrow> <mtext>B</mtext><msub> <mrow> <mo stretchy=false>(</mo><mtext>OH</mtext><mo stretchy=false>)</mo></mrow> <mn>3</mn> </msub> <mo>+</mo><mtext>NaOH</mtext><mover> <mo>⇌</mo> </mover><mtext>Na</mtext><mo stretchy=false>[</mo><mtext>B</mtext><mfenced close=] open=(> <mrow> <mtext>OH</mtext><msub> <mo stretchy=false>)</mo> <mn>4</mn> </msub></mrow></mfenced></mrow> </math> How this reaction can is made to proceed in forward direction?
Q.
How this reaction can is made to proceed in forward direction?
Solution:
forms compound with cis 1,2 diol i.e. it consumes the product so according to le chatelier principle the reaction goes in forward direction. The complex anion formed with cis 1,2 diol is stable due to formation of 5 membered ring.