- Tardigrade
- Question
- Physics
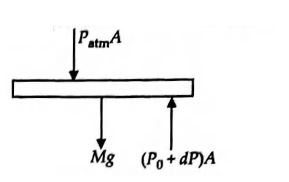
- An ideal gas enclosed in a vertical cylindrical container supports a freely moving piston of mass M . The piston and the cylinder have equal cross sectional area A. When the piston is in equilibrium, the volume of the gas is V0 and its pressure is P0 The piston is slightly displaced from the equilibrium position and released. Assuming that the system is completely isolated from its surrounding, the piston executes a simple harmonic motion with frequency
Q. An ideal gas enclosed in a vertical cylindrical container supports a freely moving piston of mass . The piston and the cylinder have equal cross sectional area . When the piston is in equilibrium, the volume of the gas is and its pressure is The piston is slightly displaced from the equilibrium position and released. Assuming that the system is completely isolated from its surrounding, the piston executes a simple harmonic motion with frequency
Solution: