- Tardigrade
- Question
- Physics
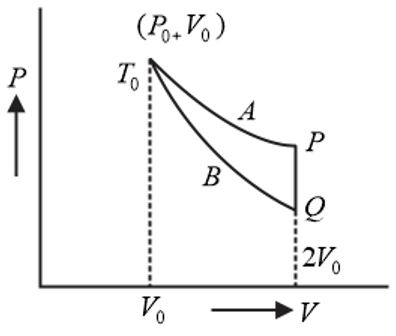
- An ideal gas ( 1 mole, monatomic) is in the initial state P (see diagram) on an isothermal curve A at a temperature T0 . It is brought under a constant volume (2 V0) process to Q which lies on an adiabatic curve B intersecting the isothermal curve A at (P0 , V0 , T0) . The change in the internal energy of the gas (in terms of T0 ) during the process is (22/3 = text1.587) <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/p-chxu9dlzb9bw.png />
Q.
An ideal gas ( mole, monatomic) is in the initial state (see diagram) on an isothermal curve at a temperature . It is brought under a constant volume process to which lies on an adiabatic curve intersecting the isothermal curve at . The change in the internal energy of the gas (in terms of ) during the process is
Solution: