Q.
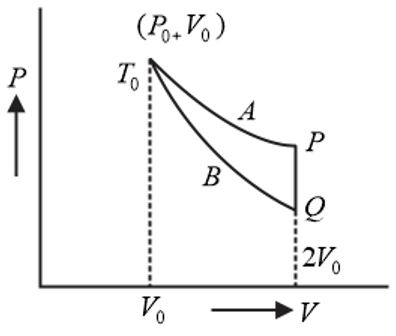
An ideal gas ( $1$ mole, monatomic) is in the initial state $P$ (see diagram) on an isothermal curve $A$ at a temperature $T_{0}$ . It is brought under a constant volume $\left(2 V_{0}\right)$ process to $Q$ which lies on an adiabatic curve $B$ intersecting the isothermal curve $A$ at $\left(P_{0} , \, V_{0} , \, T_{0}\right)$ . The change in the internal energy of the gas (in terms of $T_{0}$ ) during the process is $\left(2^{2/3} = \text{1.587}\right)$
NTA AbhyasNTA Abhyas 2020Thermodynamics
Solution: