Q.
Among the following species, identify the isostructural pairs
Solution:
For species to be isostructural they should have same hybridized state same number of and
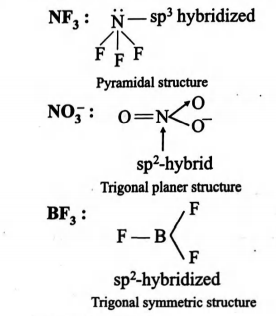
Hybridized state
L. p.
is hybridised
1
is hybridised
1
is hybridised
0
is hybridised
0
These three -hybrid orbitals are attached to each other trigonally with an angle of and they are overlapped with three -orbitals of three -atoms on their axes. Hence the geometry of molecule is trigonal planar.
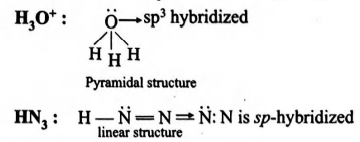
Thus isostructural pairsare and
| Hybridized state | L. p. |
|---|---|
| is hybridised |
1 | is hybridised |
1 |
| is hybridised |
0 |
| is hybridised |
0 |