Q.
Among the following species, identify the isostructural pairs
$NF_3, NO_3^-, BF_3, H_3O^+, HN_3$
Chemical Bonding and Molecular Structure
Solution:
For species to be isostructural they should have same hybridized state same number of $b.p.$ and $l.p.$
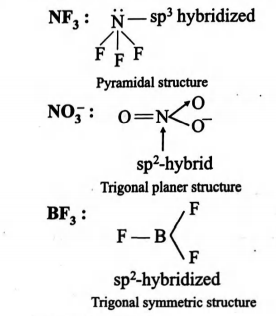
Hybridized state
L. p.
$NF_3\,\,H = \frac{1}{2} (5+3) = 4$
$\Rightarrow N$ is $sp^3$ hybridised
1
$NO^-_3\,\,H = \frac{1}{2} (5+1) = 3$
$\Rightarrow N$ is $sp^2$ hybridised
1
$BF_3\,\,H = \frac{1}{2} (3+3) = 3$
$\Rightarrow B$ is $sp^2$ hybridised
0
$B_3O^+\,\,H = \frac{1}{2} (6+3-1) = 4$
$\Rightarrow O$ is $sp^3$ hybridised
0
These three $sp^2$-hybrid orbitals are attached to each other trigonally with an angle of $120^{\circ}$ and they are overlapped with three $p$-orbitals of three $F$-atoms on their axes. Hence the geometry of $BF_3$ molecule is trigonal planar.
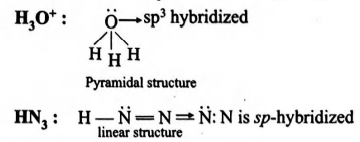
Thus isostructural pairsare $[NF_3, H_3O^+]$ and $[NO_3^-, BF_3]$
| Hybridized state | L. p. |
|---|---|
| $NF_3\,\,H = \frac{1}{2} (5+3) = 4$ $\Rightarrow N$ is $sp^3$ hybridised |
1 | $NO^-_3\,\,H = \frac{1}{2} (5+1) = 3$ $\Rightarrow N$ is $sp^2$ hybridised |
1 |
| $BF_3\,\,H = \frac{1}{2} (3+3) = 3$ $\Rightarrow B$ is $sp^2$ hybridised |
0 |
| $B_3O^+\,\,H = \frac{1}{2} (6+3-1) = 4$ $\Rightarrow O$ is $sp^3$ hybridised |
0 |