- Tardigrade
- Question
- Physics
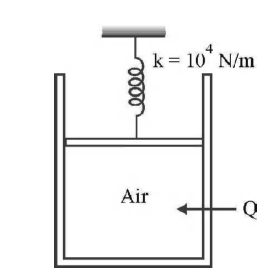
- Air is contained in a piston - cylinder arrangement as shown in Fig. with a cross - sectional area of 4 cm2 and an initial volume of 20 cc. The air is initially at a pressure of 1 atm and temperature of 20°C. The piston is connected to a spring whose spring constant is k = 104 N/m, and the spring is initially undeformed. How much heat (in joule) must be added to the air to increase the pressure to 3 atm. (For air, CV— 718 J/kg°C, molecular mass of air 28.97) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/8cbb28c053aa64958039cbfe62a29cfa-.png />
Q.
Air is contained in a piston - cylinder arrangement as shown in Fig. with a cross - sectional area of cm and an initial volume of . The air is initially at a pressure of and temperature of . The piston is connected to a spring whose spring constant is , and the spring is initially undeformed. How much heat (in joule) must be added to the air to increase the pressure to atm. (For air, , molecular mass of air )
Answer: 12.74
Solution: