Q.
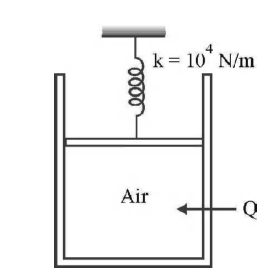
Air is contained in a piston - cylinder arrangement as shown in Fig. with a cross - sectional area of $4$ cm$^{2}$ and an initial volume of $20\, cc$. The air is initially at a pressure of $1\, atm$ and temperature of $20^{\circ}C$. The piston is connected to a spring whose spring constant is $k = 10^4 \,N/m$, and the spring is initially undeformed. How much heat (in joule) must be added to the air to increase the pressure to $3$ atm. (For air, $C_{V}— 718 J/kg^{\circ}C$, molecular mass of air $28.97$)
Thermodynamics
Solution: