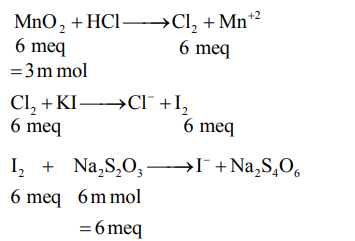- Tardigrade
- Question
- Chemistry
- A 2.0 g sample containing MnO 2 is treated with HCl liberating Cl 2. The Cl 2 gas is passed into a solution of KI and 60.0 mL of 0.1 M Na 2 S 2 O 3 is required to titrate the liberated iodine. The percentage of MnO 2 in the sample is (Nearest integer) [Atomic masses (in u) Mn =55 ; Cl =35.5 ; O =16, I =127, Na =23, K =39, S =32]
Q.
A sample containing is treated with liberating . The gas is passed into a solution of KI and of is required to titrate the liberated iodine. The percentage of in the sample is _______(Nearest integer)
[Atomic masses (in u) ,
Answer: 13
Solution: