- Tardigrade
- Question
- Chemistry
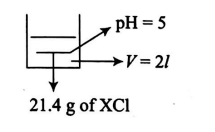
- 21.4 g XCl salt is dissolved in water and formed 2 litres of solution. The pH of the resultant solution was found to be 5 . Calculate the density (in g / cc ) of solid salt XCl, if XCl forms NaCl type crystal having R X +(radius of X +) =0.8 mathringA and R Cl -(radius of Cl -) =1.7 mathringA. Given: K b ( XOH )=2 × 10-5 ; N A =6 × 1023 Use 10-0.7= 0.20, log 2=0.3
Q.
salt is dissolved in water and formed litres of solution. The of the resultant solution was found to be Calculate the density (in ) of solid salt , if forms type crystal having (radius of ) and (radius of ) .
Given: Use
Answer: 2.85
Solution: