Q.
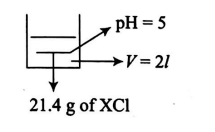
$21.4 \,g\, XCl$ salt is dissolved in water and formed $2$ litres of solution. The $pH$ of the resultant solution was found to be $5 .$ Calculate the density (in $g / cc$ ) of solid salt $XCl$, if $XCl$ forms $NaCl$ type crystal having $R _{ X ^{+}}$(radius of $X ^{+}$) $=0.8 \,\mathring{A}$ and $R _{ Cl ^{-}}$(radius of $Cl ^{-}$) $=1.7 \,\mathring{A}$.
Given: $K _{ b }( XOH )=2 \times 10^{-5} ; N _{ A }=6 \times 10^{23}$ Use $10^{-0.7}=$ $0.20, \log 2=0.3$
The Solid State
Solution: