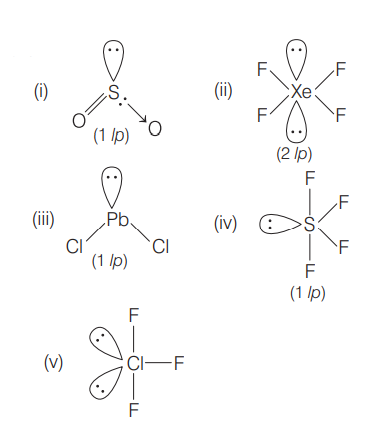
Q.
Which set of the following molecules has only one lone pair of electrons on their respective central atoms?
a) $SO_2$
b) $XeF_4$
c) $P b Cl_2$
d) $SF_4$
e) $ClF_3$
AP EAMCETAP EAMCET 2018
Solution: