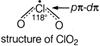
Q. Which one of the following statement is wrong about $ Cl{{O}_{2}} $ ?
BVP MedicalBVP Medical 2013
Solution:
Option is wrong $ Cl{{O}_{2}} $ molecule is paramagnetic due to the presence of one odd electron in a p-orbital. $ Cl{{O}_{2}} $ do not dimerize because odd electron is delocalised.