Q. Which one of the following plots is true for the first order decomposition of $ N_{2}O_{5} $ ?
AMUAMU 2000
Solution:
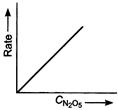
If a straight line is obtained in rate versus concentration graph, then the reaction is of first order, ie,
Rate $=k[R]$
In the following graph, the graph $(d)$ is true for the first order, decomposition of $N _{2} O _{5}$. In this graph a straight line is obtained when it is plotted rate versus concentration, ie,
Rate $=k\left[ N _{2} O _{5}\right]$
Rate of decomposition of $N _{2} O _{5}$ as a function of $\left[ N _{2} O _{5}\right]$.