Q. Which of the following will have a meso-isomer also?
Jharkhand CECEJharkhand CECE 2008
Solution:
[a] $ =C{{H}_{3}}-\underset{Cl}{\mathop{\underset{|}{\mathop{\overset{*}{\mathop{C}}\,}}\,}}\,H-C{{H}_{2}}C{{H}_{3}} $
One asymmetric carbon atom, forms d- and $ l- $ optical isomers.
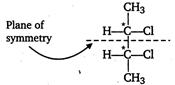
Meso due to internal compensation
[b] Two asymmetric carbon atoms, forms $ d-,l- $ and meso forms.
[c] $ C{{H}_{3}}-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-\underset{Cl}{\mathop{\underset{|}{\overset{*}{\mathop{CH}}}\,}}\,-C{{H}_{2}}C{{H}_{3}} $
Two asymmetric carbon atoms but does not have symmetry. Hence, meso form is not formed.
[d] $ C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\overset{*}{\mathop{C}}}\,}}\,H-COOH $
One asymmetric carbon atom, meso form is not formed.