Q.
Which of the following structures contain $ sp $ -hybridised carbon atom $ (s) $ ?
I. $ HC \equiv CH $
II. $ H _{2}C = C = CH_{2} $
III. 
Iv. 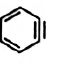
AMUAMU 2018Organic Chemistry – Some Basic Principles and Techniques
Solution:

 both carbon are $sp$-hybridised
both carbon are $sp$-hybridised  central carbon is $sp$- hybridised
central carbon is $sp$- hybridised 