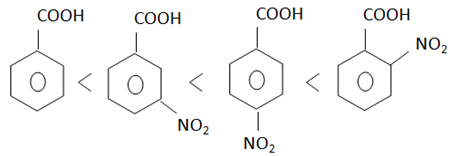
Q.
Which of the following is correct order for acidity of the given carboxylic acids?
$\left(A\right)PhCOOH$
$\left(B\right)o-NO_{2}C_{6}H_{4}COOH$
$\left(C\right)p-NO_{2}C_{6}H_{4}COOH$
$\left(D\right)m-NO_{2}C_{6}H_{4}COOH$
NTA AbhyasNTA Abhyas 2020Organic Chemistry – Some Basic Principles and Techniques
Solution: