Q. Which of the following has square planar geometry?
BITSATBITSAT 2010
Solution:
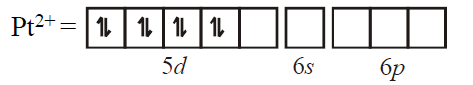
$\left[ PtCl _{4}\right]^{2-}$ has square planar geometry. $Pt : 5 d^{9} 6 s^{1}$ Two electrons are removed from $5 d$ shell and $6\, s$ shell. So, hybridisation takes place is $d s p^{2}$ i.e. square planar geometry.