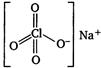
Q. Which of the following compounds of chlorine contains both ionic and covalent bonds?
AMUAMU 2009
Solution:
$NaClO _{4}$ contains both ionic and covalent bonds. $Na$ is highly electropositive and $ClO _{4}^{-}$is highly electronegative, so they will form ionic bond. Perchlorate ion, $ClO _{4}^{-}$has a tetrahedral structure with $s p^{3}$ hybridisation. $Cl$ and $O$ are bonded through covalent bonds.