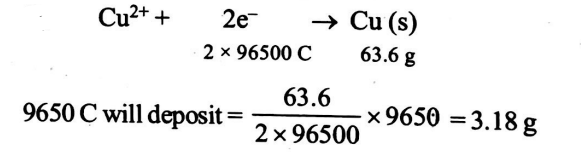Q. When $ 9650$ coulombs of electricity is passed through a solution of copper sulphate, the amount of copper deposited is (given at. wt. of $Cu =63.6$ )
Electrochemistry
Solution:
Correct answer is (b) $3.18 \,g$