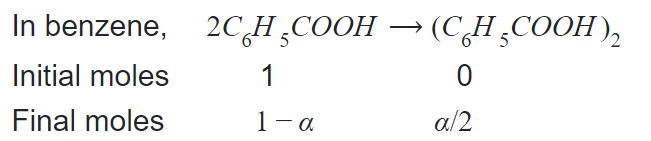Q. When $2.44$ grams of benzoic acid $\left( C _{6} H _{5} COOH \right)$ dissolved in $25$ grams of benzene, it shows depression of freezing point equal to $2.2\, K$. Molal depression constant of benzene is $5.0\, K\,kg \,mol ^{-1}$. What is the percentage association of acid, if it forms dimer in solution?
TS EAMCET 2020
Solution: