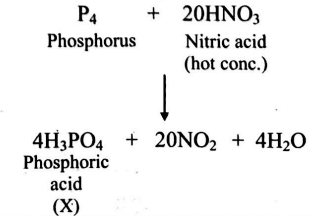
Q.
What is the molecular mass (in amu) of the product ' $X$ ' in the following unbalanced chemical equation?
$P _{4}+ \underset{\text{(Hot conc)}}{HNO _{3}} \longrightarrow X + NO _{2}+ H _{2} O$
[Given At. Wt.: $P=31\, u , H=1\, u , N =14\, u, O =16\, u$ ]
The p-Block Elements - Part2
Solution: