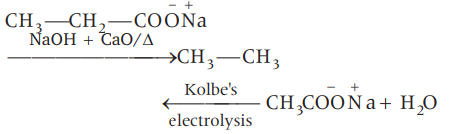Q.
What are $X. Y$ and $Z$ in the following reations?
$CH _{2}- CH _{2}- CO\overset{\ominus}{O} \overset{\oplus}{N}a \ce{->[{X}]} Y \ce{<-[{\text{Kolbe's Electrolysis}}]}Z + H_2 O $
X
Y
Z
(a)
$NaOH + CaO / \Delta$
$CH _{3} CH _{2} CH _{2} CH _{3}$
$CH _{3} CH _{2} CO\overset{\ominus}{O}\overset{\oplus}{N}a$
(b)
$MO _{2} O _{3}$
$C _{2} H _{6}$
$CH _{3} CH _{2} CO\overset{\ominus}{O}\overset{\oplus}{N}a$
(c)
$NaOH + CaO / \Delta C _{2} H _{6}$
$C _{2} H _{6}$
$CH _{3}CO\overset{\ominus}{O}\overset{\oplus}{N}a$
(d)
$\left( CH _{3} COO \right)_{2} Mn / \Delta C _{3} H _{8}$
$C _{3} H _{8}$
$CH _{3}CH_2 CO\overset{\ominus}{O}\overset{\oplus}{N}a$
| X | Y | Z | |
| (a) | $NaOH + CaO / \Delta$ | $CH _{3} CH _{2} CH _{2} CH _{3}$ | $CH _{3} CH _{2} CO\overset{\ominus}{O}\overset{\oplus}{N}a$ |
| (b) | $MO _{2} O _{3}$ | $C _{2} H _{6}$ | $CH _{3} CH _{2} CO\overset{\ominus}{O}\overset{\oplus}{N}a$ |
| (c) | $NaOH + CaO / \Delta C _{2} H _{6}$ | $C _{2} H _{6}$ | $CH _{3}CO\overset{\ominus}{O}\overset{\oplus}{N}a$ |
| (d) | $\left( CH _{3} COO \right)_{2} Mn / \Delta C _{3} H _{8}$ | $C _{3} H _{8}$ | $CH _{3}CH_2 CO\overset{\ominus}{O}\overset{\oplus}{N}a$ |
AP EAMCETAP EAMCET 2018
Solution: