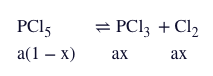Q. Two moles of $PCl_{5}$ were heated in a closed vessel of 2 L. At equilibrium 40% of $PCl_{5}$ is dissociated into $PCl_{3} \, and \, Cl_{2}$ . The value of equilibrium constant is
NTA AbhyasNTA Abhyas 2020Equilibrium
Solution: