Q.
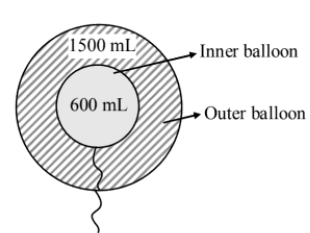
Two inflated balloons I and II (thin skin) having volume $600\,ml$ and $1500\,ml$ at $300\,K$ are taken as shown in diagram. If maximum volume of inner and outer balloons are $800\,ml$ and $1800\,ml$ respectively then find the balloon which will burst first on gradual heating.
NTA AbhyasNTA Abhyas 2022
Solution: