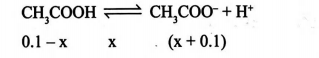Q. To $100 \,mL$ of an aqueous solution of $0.1 M CH _{3} COOH$ $\left( K _{ a }=2 \times 10^{-5}\right), 0.01 mol$ of $HCl ( g )$ is passed. Select correct options regarding the resulting solution.
Equilibrium
Solution: