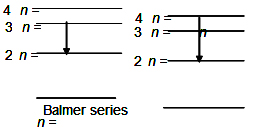Q. The wavelength of the first spectral line in the Balmer series of the hydrogen atom is $6561 \, \overset{^\circ }{A}$ . The wavelength of the second spectral line in the Balmer series of singly ionized helium atom is
NTA AbhyasNTA Abhyas 2020Atoms
Solution: