Q.
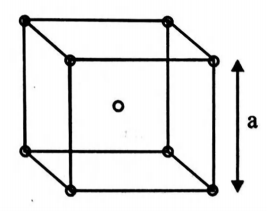
The unit cell in a body centred cubic lattice is given in the figure. Each sphere has a radius $r$ and the cube has side $'a'.$ What fraction of the total cube volume is empty?
The Solid State
Solution: