Q. The type of magnetism exhibited by$ [Mn(H_2O )_6]^{2+}$ ion i s .......
IIT JEEIIT JEE 1994Coordination Compounds
Solution:
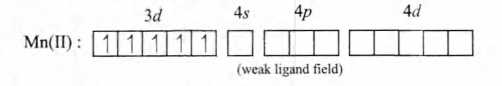
Paramagnetism: In $[Mn(H_2O)_6]^{2+}, Mn(II)$ has $3d^5$ configuration. Since, $H_2O$ is a weak ligand, all five $d$-electrons are unpaired :