Q. The structure of diamagnetic nickel complex, $\left[N i(C N)_{4}\right]^{2-}$ is
AMUAMU 2013
Solution:
In $\left[N i(C N)_{4}\right]^{2-}$,
nickel is in $+2$ oxidation state
and has electronic configuration $3 d^8$.
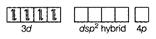
Orbitals of $N i^{2+}$ ion
$d s p^{2}$ hybridised orbitals of $N i^{2+}$
$\left[N i(C N)_{4}\right]^{2-}$ low spin complex
Therefore, the shape is square planar and the hybridisation involved is $d s p^{2}$.