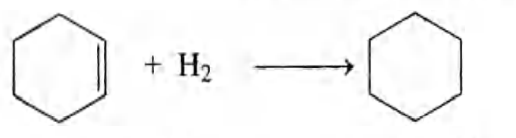Q. The standard enthalpy of combustion at $25^{\circ} C$ of hydrogen, cyclohexene $\left( C _{6} H _{10}\right)$ and cyclohexane $\left( C _{6} H _{12}\right)$ are $-241, -3800$ and $-3920\, kJ / mol$ respectively. Calculate the heat of hydrogenation of cyclohexene.
IIT JEEIIT JEE 1989Thermodynamics
Solution: