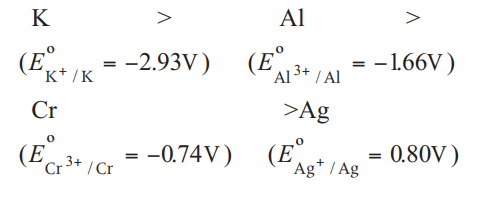Q. The standard electrode potential $\left(E^{-}\right)$values of $-1.66\, V , 0.80\, V , 2.93\, V$ and $-0.74\, V$, respectively. The correct decreasing order of reducing power of the metal is
Redox Reactions
Solution: