Q.
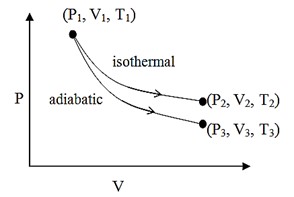
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement is incorrect?
NTA AbhyasNTA Abhyas 2022
Solution: