Q.
The progress of the reaction, $P \rightarrow n Q$ with time is represented in the graph shown below.
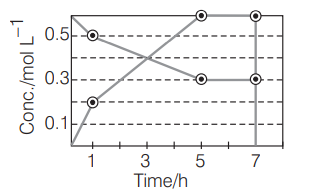
The initial rate of conversion of $P$ will be
Chemical Kinetics
Solution: