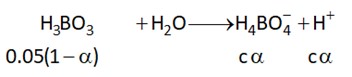Q.
The $pH$ at the equivalence point for the titration of $0.10 \, M$ $KH_{2}BO_{3}$ with $0.1 \, M \, HCl$ is (Report the answer in the nearest integer value)
$\left(K_{a} \, of \, H_{3} \left(BO\right)_{3} = 12 . 8 \left(\times 10\right)^{- 10}\right)$
NTA AbhyasNTA Abhyas 2022
Solution: