Q. The pair having the same magnetic moment is [atomic number, $Cr =24, Mn =25, Fe =26$ and $Co =27]$
Coordination Compounds
Solution:
The complexes, in which metals ions have same number of unpaired electrons will have same magnetic moment.
Complex ion
Electronic configuration of metal ion
Number of unpaired electrons $(n)$
$\left[ Cr \left( H _{2} O \right)_{6}\right]^{2+}$
$Cr ^{2+} ;[ Ar ] 3 d^{4}$

$\left[ Fe \left( H _{2} O \right)_{6}\right]^{2+}$
$Fe ^{2+} ;[ Ar ] 3 d^{6}$

$\left[ Mn \left( H _{2} O \right)_{6}\right]^{2+}$
$Mn ^{2+} ;[ Ar ] 3 d^{5}$

$\left[ CoCl _{4}\right]^{2-}$
$Co ^{2+} ;[ Ar ] 3 d^{7}$
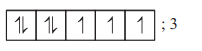
| Complex ion | Electronic configuration of metal ion | Number of unpaired electrons $(n)$ |
|---|---|---|
| $\left[ Cr \left( H _{2} O \right)_{6}\right]^{2+}$ | $Cr ^{2+} ;[ Ar ] 3 d^{4}$ |  |
| $\left[ Fe \left( H _{2} O \right)_{6}\right]^{2+}$ | $Fe ^{2+} ;[ Ar ] 3 d^{6}$ |  |
| $\left[ Mn \left( H _{2} O \right)_{6}\right]^{2+}$ | $Mn ^{2+} ;[ Ar ] 3 d^{5}$ |  |
| $\left[ CoCl _{4}\right]^{2-}$ | $Co ^{2+} ;[ Ar ] 3 d^{7}$ |  |