Q.
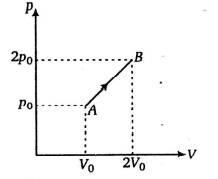
The $p-V$ diagram of $2 g$ of helium gas for a certain process $A \rightarrow B$ is shown in the figure. What is the heat given to the gas during the process $A \rightarrow B$ ?
AFMCAFMC 2011
Solution: