Q. The optically active coordination complex ion among the following is
J & K CETJ & K CET 2009Coordination Compounds
Solution:
Usually optical isomerism is exhibited by
$\left[M(a a)_{3}\right]$ or $\left[M(a a)_{2} X_{2}\right]$
or $\left[M(a a) X_{2} 1 / 2\right]$ type complexes.
(Here $M =$ metal atom, $a a=$ bidentate ligand $X$, and $Y =$ unidentate ligand).
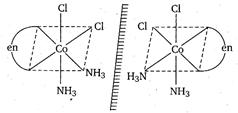
Hence, $cis\left[ Co \left(e n\left(N H_{3}\right)_{2} Cl _{2}\right]^{+}\right.$ exhibits optical isomerism. Its optically active forms are as:
Only cis form of tetrahedral complexes of the type $\left[M(a a)_{2 \times 2}\right]^{+}$is optically active.