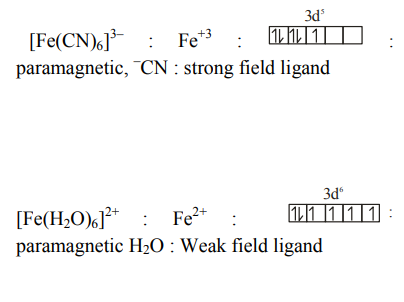Q.
The number of paramagnetic species from the following is ________
$ {\left[ Ni ( CN )_4\right]^{2-} \cdot\left[ Ni ( CO )_4\right],\left[ NiCl _4\right]^{2-}} $
$ {\left[ Fe ( CN )_6\right]^{4-},\left[ Cu \left( NH _3\right)_4\right]^{2+}}$
$ {\left[ Fe ( CN )_6\right]^{3-} \text { and }\left[ Fe \left( H _2 O \right)_6\right]^{2+}}$
Solution: