Q. The molecular shapes of $ SF_4 $ , $ CF_4 $ and $ XeF_4 $ are
UPSEEUPSEE 2007
Solution:
Molecule
Structure
Hybridisation
of central atom
Lone
pair
$SF_4$

$sp^3d$
One
$CF_4$
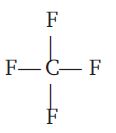
$sp^3$
zero
$XeF_4$

$sp^3d^2$
two
| Molecule | Structure | Hybridisation of central atom | Lone pair |
|---|---|---|---|
| $SF_4$ |  |
$sp^3d$ | One |
| $CF_4$ | 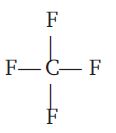 |
$sp^3$ | zero |
| $XeF_4$ |  |
$sp^3d^2$ | two |