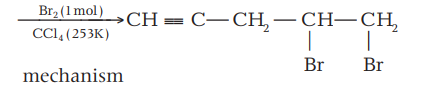Q.
The major product $(P)$ formed in the below reaction is
$H - C \equiv C - CH _{2}- CH = CH _{2} \ce{ ->[{Br_2 (1 mol)}][{CCl_4,253 K}] }P$
TS EAMCET 2018
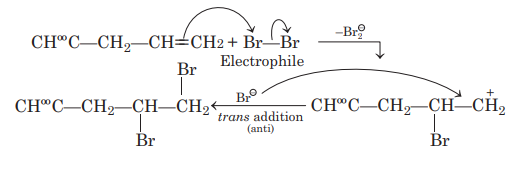
Solution: