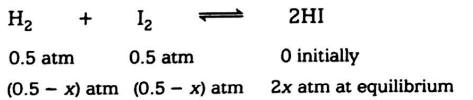
Q. The $ K_{p} $ value for the reaction, $ H_{2} + I_{2} {\rightleftharpoons} 2HI $ at $ 460^{\circ}C $ is $ 49 $ . If the initial pressure of $ 2 $ and $ 2 $ is $ 0.5 $ atm, respectively, what will be the partial pressure of $ H_{2} $ at equilibrium?
AMUAMU 2014Equilibrium
Solution: